Technology ≫
Why Choose Quantitative Phase Imaging(QPI)?
HTS & HCS
A Dual Revolution
in Cell Analysis

High Throughput Screening (HTS): From Manual to Automatic
In the late 1980s, the first high-throughput screening equipment emerged. High-throughput screening is based on experimental methods at the molecular and cellular levels, using microplate formats as experimental tool carriers, and executing experimental processes through automated operating systems. This technology collects experimental result data through sensitive and fast detection instruments and uses computers for analysis and processing, enabling the detection of tens of millions of samples at the same time.

High-Content Screening (HCS): From Single-Dimensional to Multi-Dimensional
In 1997, the first fully integrated high-content imaging platform was launched. High-content screening is a high-throughput screening method that detects the effects of screened samples on various attributes and physiological states of cells (such as cell morphology, growth and differentiation status, migration and apoptosis, cell metabolic pathways, and various links of signal pathways) while maintaining the integrity of cell structure and function.

Label-Free High Content: From Labeled to Label-Free
In contrast, traditional label-free imaging technologies are difficult to perform accurate quantitative analysis, which limits their application in high-content screening scenarios. Quantitative phase imaging is expected to fill the gap of label-free imaging in the field of high-content screening.
From “Labeled” to “Label-Free”: The Evolution and Transcendence of Cell Imaging
Labeled
Label-Free

Bright-Field Imaging
Bright-field imaging presented the microscopic world to people, making life sciences visible. However, for transparent or semi-transparent samples, the contrast provided by bright-field imaging is low, making it difficult to distinguish sample details.

Bright-Field Staining
Bright-field staining can significantly improve the contrast of samples under a bright-field microscope by using specific dyes to react specifically with different components in cells, allowing direct observation of cell morphology and structure under different colors. But the staining process may cause cell damage or even death.

Phase-Contrast Imaging
Phase-contrast imaging converts the optical path difference of light passing through the sample into amplitude difference, solving the problem of low contrast of transparent samples under ordinary bright-field microscopes without staining. However, for highly transparent samples or samples with small phase changes, the contrast is still limited, and quantitative analysis cannot be performed.

Fluorescence Imaging
Fluorescence imaging uses fluorescent dyes or fluorescent probes to label samples, which can provide high-contrast images. Compared with bright-field staining, it causes less damage to samples and can quantify target molecules through fluorescence intensity. However, it has the disadvantages of phototoxicity and photobleaching.

Differential Interference Contrast (DIC) Imaging

Confocal Fluorescence Imaging

Digital Holographic Imaging

Super-Resolution Fluorescence Imaging

Quantitative Phase Imaging
Interferometric Quantitative
Phase Imaging
It recovers phase information by allowing an object light wave passing through the sample to interfere with a reference light wave, generating an interference pattern, and analyzing these interference fringes.
Non-Interferometric Quantitative
Phase Imaging
It recovers phase information by analyzing the light intensity distribution, but generally, it is not as good as the interferometric method in terms of absolute accuracy.
HTS & HCS
A Dual Revolution
in Cell Analysis

High Throughput Screening (HTS): From Manual to Automatic
In the late 1980s, the first high-throughput screening equipment emerged. High-throughput screening is based on experimental methods at the molecular and cellular levels, using microplate formats as experimental tool carriers, and executing experimental processes through automated operating systems. This technology collects experimental result data through sensitive and fast detection instruments and uses computers for analysis and processing, enabling the detection of tens of millions of samples at the same time.

High-Content Screening (HCS): From Single-Dimensional to Multi-Dimensional
In 1997, the first fully integrated high-content imaging platform was launched. High-content screening is a high-throughput screening method that detects the effects of screened samples on various attributes and physiological states of cells (such as cell morphology, growth and differentiation status, migration and apoptosis, cell metabolic pathways, and various links of signal pathways) while maintaining the integrity of cell structure and function.

Label-Free High Content: From Labeled to Label-Free
In contrast, traditional label-free imaging technologies are difficult to perform accurate quantitative analysis, which limits their application in high-content screening scenarios. Quantitative phase imaging is expected to fill the gap of label-free imaging in the field of high-content screening.
From “Labeled” to “Label-Free”: The Evolution and Transcendence of Cell Imaging
Labeled
Label-Free

1590 Bright-Field Imaging
Bright-field imaging presented the microscopic world to people, making life sciences visible. However, for transparent or semi-transparent samples, the contrast provided by bright-field imaging is low, making it difficult to distinguish sample details.

1850 Bright-Field Staining
Bright-field staining can significantly improve the contrast of samples under a bright-field microscope by using specific dyes to react specifically with different components in cells, allowing direct observation of cell morphology and structure under different colors. But the staining process may cause cell damage or even death.

1935 Phase-Contrast Imaging
Phase-contrast imaging converts the optical path difference of light passing through the sample into amplitude difference, solving the problem of low contrast of transparent samples under ordinary bright-field microscopes without staining. However, for highly transparent samples or samples with small phase changes, the contrast is still limited, and quantitative analysis cannot be performed.

1951 Fluorescence Imaging
Fluorescence imaging uses fluorescent dyes or fluorescent probes to label samples, which can provide high-contrast images. Compared with bright-field staining, it causes less damage to samples and can quantify target molecules through fluorescence intensity. However, it has the disadvantages of phototoxicity and photobleaching.

1952 Differential Interference Contrast (DIC) Imaging
Differential interference contrast imaging uses polarized light and differences in sample thickness or optical density to enhance contrast, which can highlight details of the sample surface and internal structure. But it is mainly used for qualitative analysis and still difficult to perform accurate quantitative measurement.

1950s Confocal Fluorescence Imaging
Confocal fluorescence imaging achieves near-diffraction-limit resolution imaging by restricting out-of-focus stray light through a pinhole conjugate to the sample plane. However, it also has the problems of phototoxicity and photobleaching, and its point-by-point scanning method results in relatively slow imaging speed.

1990s Digital Holographic Imaging
Digital holographic imaging uses photoelectric sensors to record holograms and realizes holographic reconstruction and processing of samples by simulating optical diffraction processes with computers. It can provide phase information to quantify physical parameters such as the optical thickness and refractive index of samples, but complex digital processing algorithms are required to reconstruct images.

21st Century Super-Resolution Fluorescence Imaging
Super-resolution fluorescence imaging can break through the optical diffraction limit, but it has slow imaging speed and complex data processing, and still cannot avoid the impact of labeling on cell viability.

21st Century Quantitative Phase Imaging
Quantitative phase imaging technology does not require cell labeling or fluorescent probes. It realizes high-contrast quantitative imaging by reconstructing the phase information of the light field. While maintaining cell viability, it can in-depth analyze cell structure, dynamic behavior, and extract key biological information such as cell dry mass and mechanical properties. It is a technology that combines the advantages of label-free imaging and high-content analysis, providing a non-invasive long-term monitoring method for live cell research.
Non-Interferometric Quantitative Phase Imaging
It recovers phase information by analyzing the light intensity distribution, but generally, it is not as good as the interferometric method in terms of absolute accuracy.
Interferometric Quantitative Phase Imaging
It recovers phase information by allowing an object light wave passing through the sample to interfere with a reference light wave, generating an interference pattern, and analyzing these interference fringes.
Principle of Interferometric Quantitative Phase Microscopy
The label-free imaging technology adopted by BJR is interferometric Quantitative Phase Microscopy (iQPM). Using the principle of light interference, one detection light passes through the sample, and the other is a reference light that is not affected by the sample. They interfere at the imaging plane to form an interferogram; then, the phase information of the sample is extracted from the interference pattern through mathematical algorithms, and finally, the phase distribution map of the sample is obtained, thereby revealing the internal structure and composition of the sample in a non-invasive way. It is especially suitable for the study of transparent or semi-transparent biological samples.
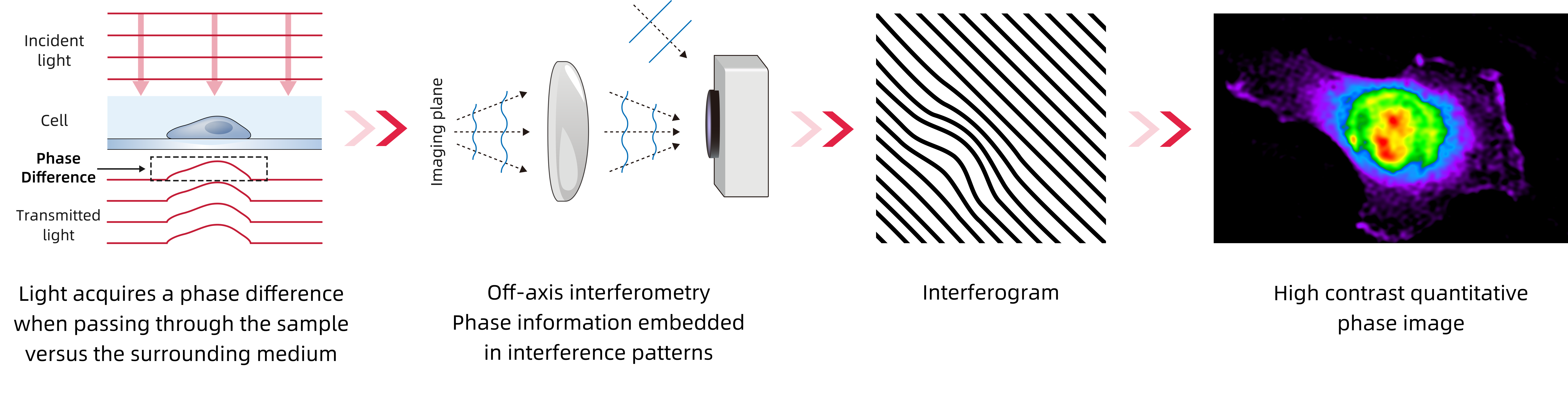
What is Phase?
Phase refers to the position of a wave in its cycle at a specific moment: a scale indicating whether it is at the peak, trough, or a certain point in between. When light passes through or reflects from different media, its speed changes due to the different refractive indices of the media, resulting in a change in the phase of the light wave.
In quantitative phase imaging, phase refers to the relative phase difference between the sample light and the background light. This phase difference can provide rich information about the internal structure and composition of the sample, such as the distribution of organelles in cells and changes in cell membrane thickness.
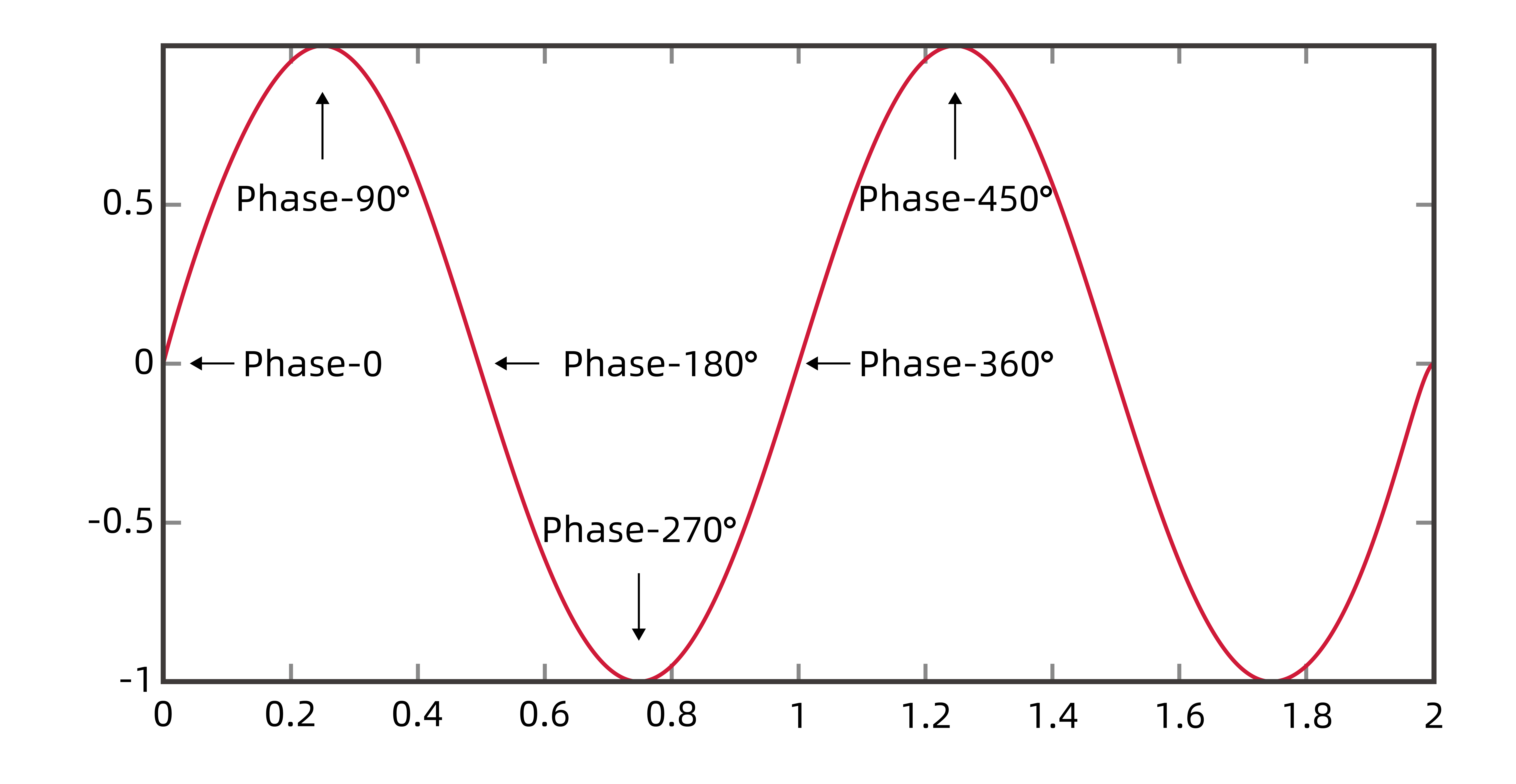
Phase Diagram
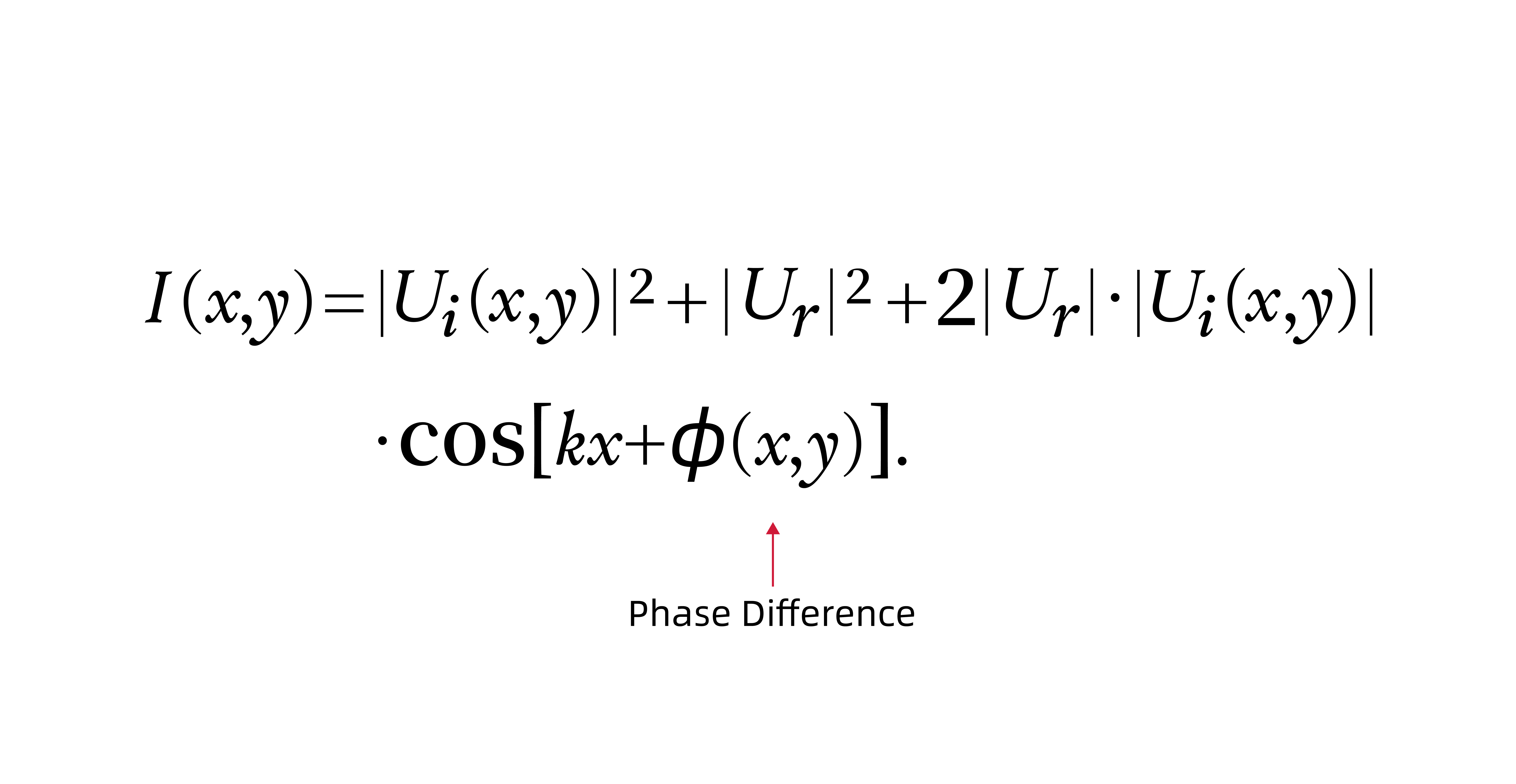
Phase difference between object light and reference light in interference
Interferometric Quantitative Phase Microscopy – An Innovative Technology
Based on Three Nobel Prizes

1953 Phase-contrast Microscopy
Phase-contrast microscopy technology first used phase information in imaging, solving the problem of low contrast when traditional microscopes observe transparent objects.

1971 Holography
The core breakthrough of holography – recording and reproducing the phase information of light waves, providing a basic framework for subsequent quantitative phase imaging.

2009 Digital Camera Sensor Technology & Laser Technology
The charge-coupled device (CCD) invented by Boyle and Smith is crucial for the detection of weak phase changes in quantitative phase imaging technology.
Interferometric Quantitative Phase Microscopy – An Innovative Technology
Based on Three Nobel Prizes

Phase-contrast Microscopy
Phase-contrast microscopy technology first used phase information in imaging, solving the problem of low contrast when traditional microscopes observe transparent objects.

Holography
The core breakthrough of holography – recording and reproducing the phase information of light waves, providing a basic framework for subsequent quantitative phase imaging.

Digital Camera Sensor Technology & Laser Technology
The charge-coupled device (CCD) invented by Boyle and Smith is crucial for the detection of weak phase changes in quantitative phase imaging technology.